Our group is an energy material lab with specific interests in designing, fabricating, characterizing and applying advanced materials for energy conversion and storage, micro-electronics and renewable energy applications. We are interested in tracking critical questions related to advanced materials and fundamental, technological issues. Our goal is to be able to design new materials with revolutionary properties for next-generation energy applications, e.g., energy storage and conversion, as well as to contribute new insights into the chemical/material origins by applying a range of advanced material manufacturing technologies and operando characterization tools developed in our group and the field.
Overview
Calcium-ion Batteries
Calcium-ion batteries (CIBs) are emerging as a promising alternative to lithium-ion batteries due to the abundance of calcium and its dendrite-free nature. Our research aims to develop high-performance CIBs and explore their mechanisms. For the cathode, we investigate polyanionic compounds and organic small molecules, aiming to enhance energy density and stability. For the anode, we examine calcium metal and graphite intercalation compounds, focusing on electrolyte regulation to optimize capacity and cycling performance. By addressing these key issues, our research seeks to advance the development of efficient and sustainable CIBs, contributing to the future of energy storage technology.

Zinc-ion Batteries
Zinc-ion batteries (ZIBs) are gaining significant attention for large-scale energy storage systems due to their abundant zinc resources, environmental friendliness, and excellent safety profile. Our research aims to develop high-performance ZIBs and explore their underlying mechanisms. In terms of electrolytes, we investigate missing-linker defect functionalized MOFs to enhance zinc ion conductivity and improve stability. For the anode, we focus on zinc metal anodes, emphasizing interface regulation to optimize long cycle life and deep discharge performance. By addressing these critical issues, our research seeks to advance the development of efficient and sustainable ZIBs, contributing to their large-scale application in the future.

In-situ Characterization Techniques
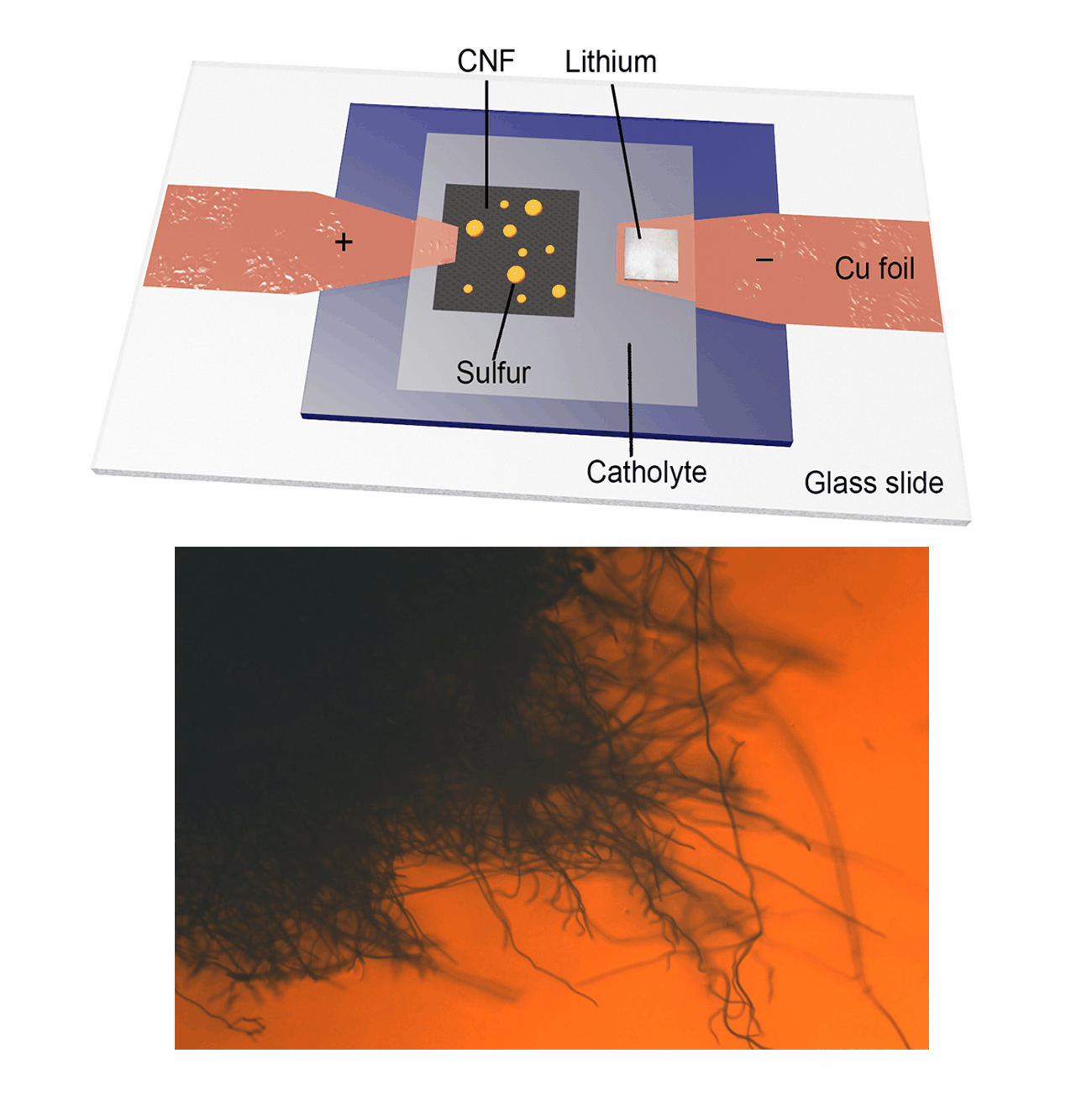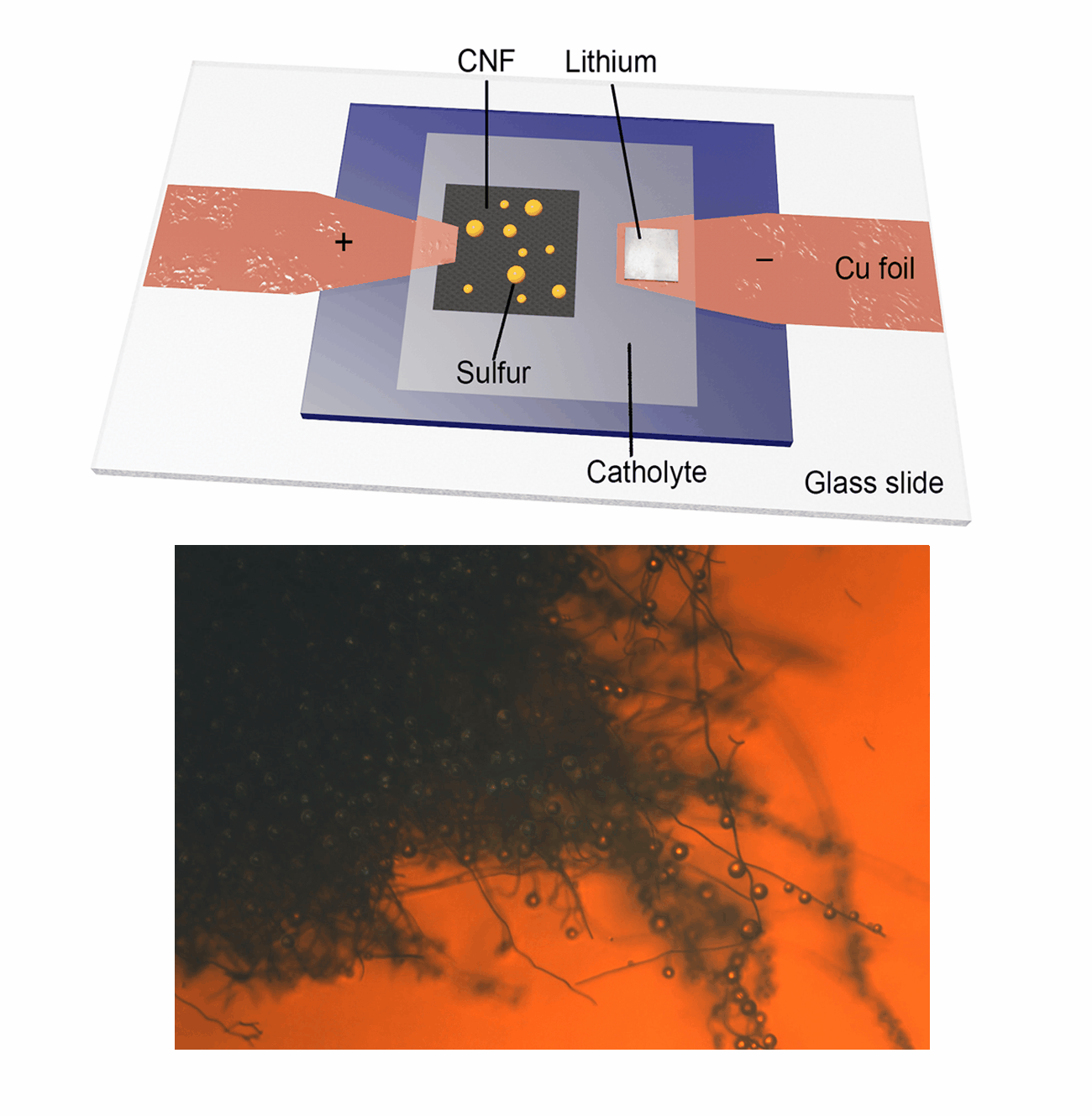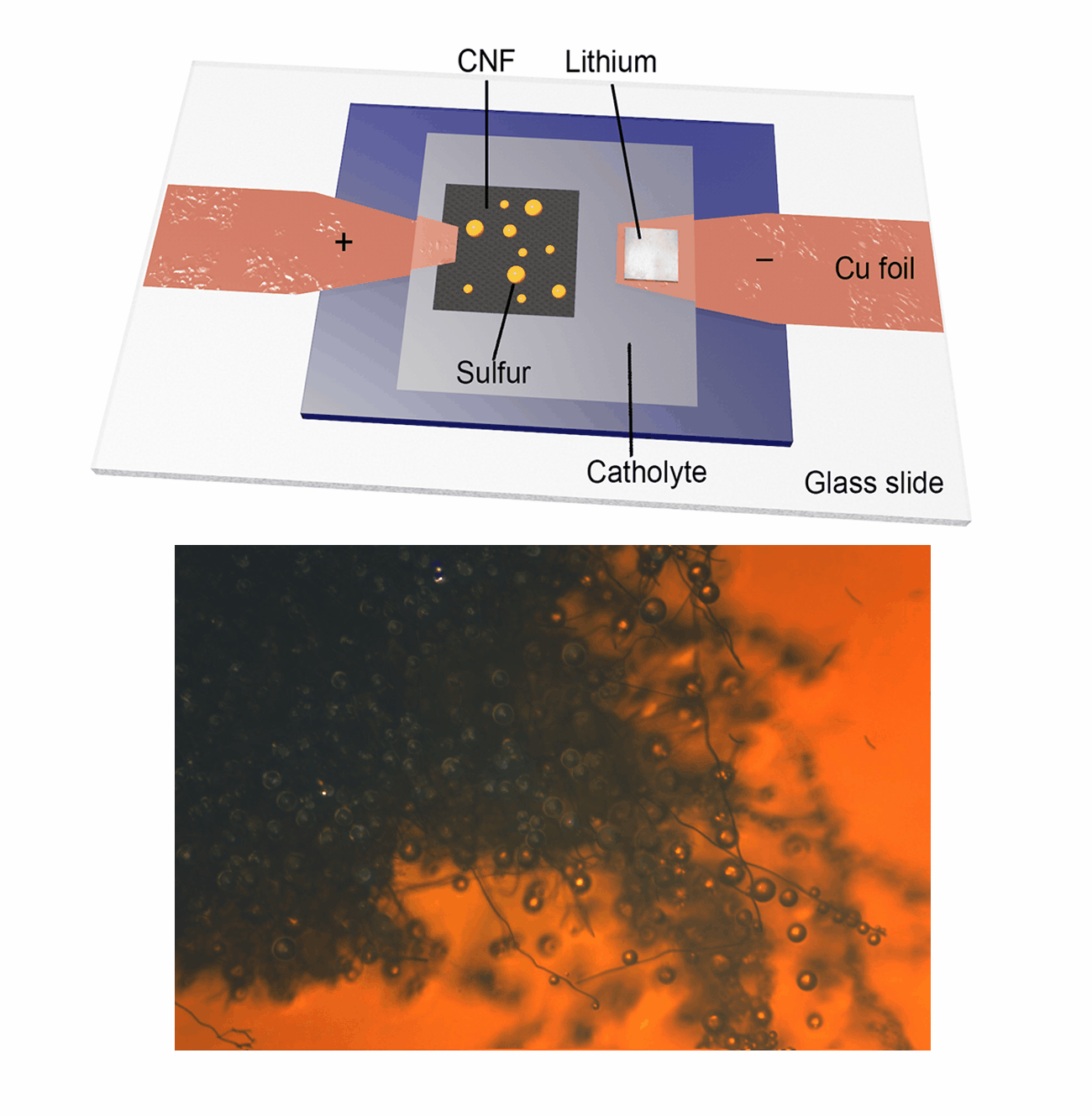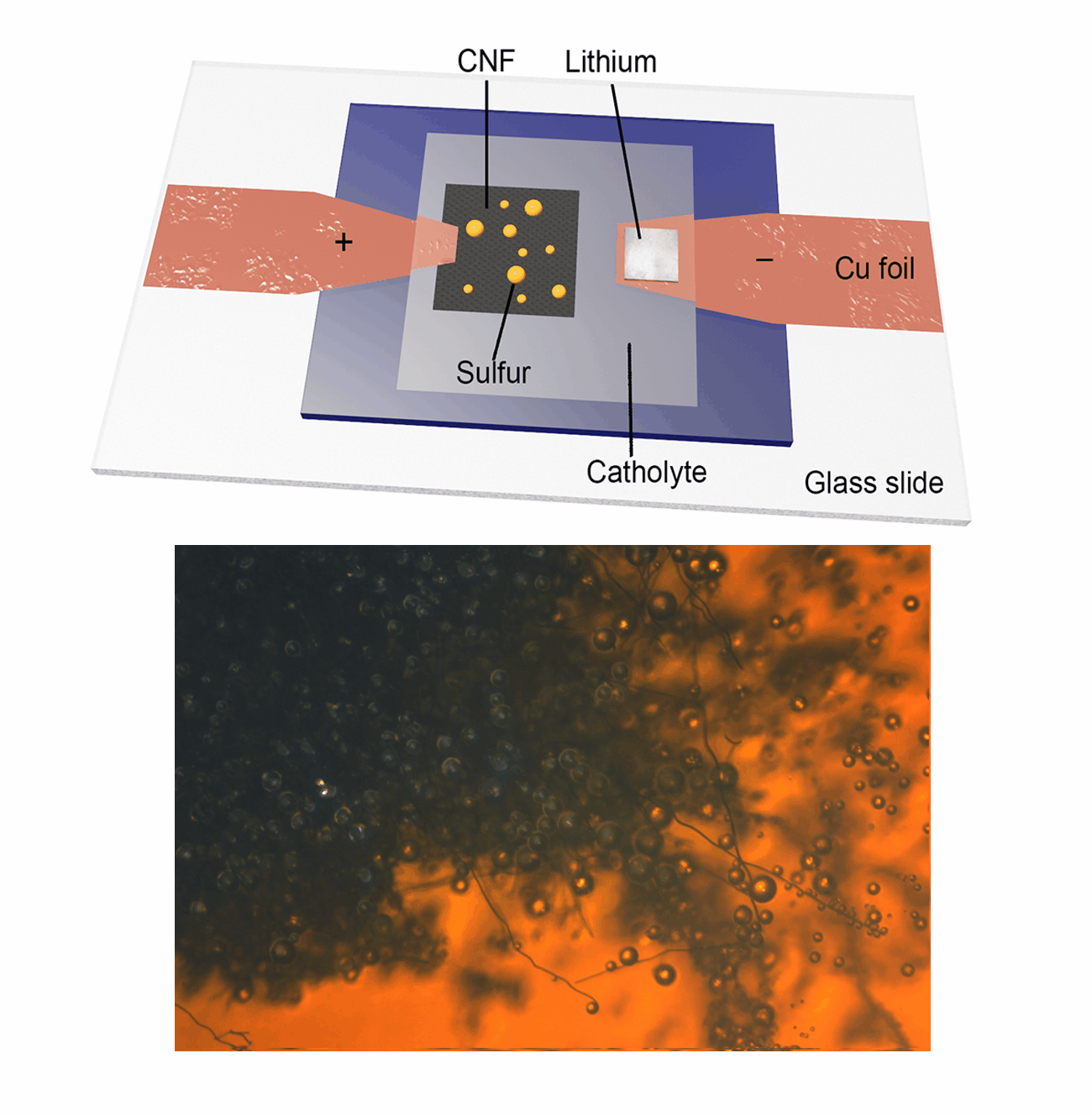
Our research group focuses on the development and application of in-situ characterization techniques as powerful platforms for probing the dynamic evolution of active materials, particularly during electrochemical reactions. Utilizing an advanced in-situ microscopy system integrated with a micro-cell device, we have achieved real-time observation of liquid sulfur droplet formation on various substrates, including three-dimensional carbon nanofibers, PAN-derived carbon films, and two-dimensional transition metal dichalcogenides. Furthermore, we have investigated the sulfur oxidation reaction chemistry under extreme conditions, such as low temperatures and low electrolyte/sulfur ratios in Li-S batteries. This innovative methodology is being extended to explore other advanced electrode materials in rechargeable battery systems, including Ca-S batteries, Ca-ion batteries, and Zn-ion batteries. Our work aims to provide fundamental insights into the electrochemical processes and material behaviors that underpin next-generation energy storage technologies.